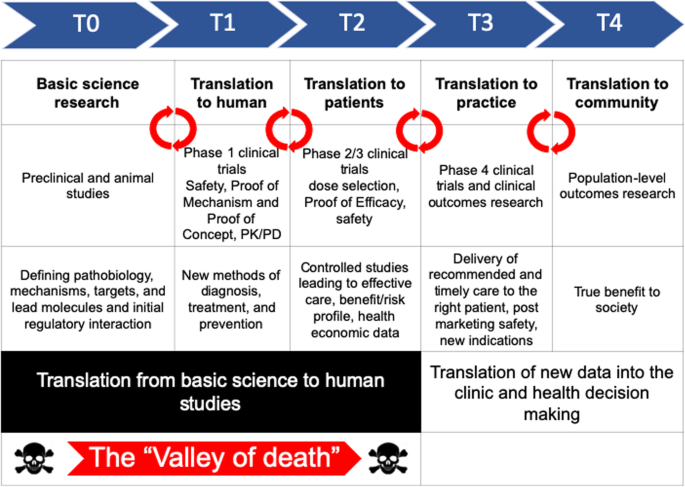
Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles | Translational Medicine Communications | Full Text

Frontiers | The Current Status and Future Direction of Clinical Research in Japan From a Regulatory Perspective







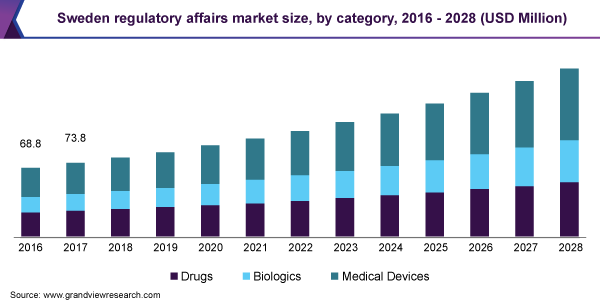


![Regulatory Affairs Market Size, Share, Growth [2023 Report] Regulatory Affairs Market Size, Share, Growth [2023 Report]](https://www.grandviewresearch.com/static/img/research/global-regulatory-affairs-market.png)






![Regulatory Affairs Market Size, Share, Growth [2023 Report] Regulatory Affairs Market Size, Share, Growth [2023 Report]](https://www.grandviewresearch.com/static/img/research/us-regulatory-affairs-market.png)