Role of Extensive Documentation in Smooth Conduct of Clinical Trials | WorkSure™ MedPharma Consultancy India Pvt. Ltd.
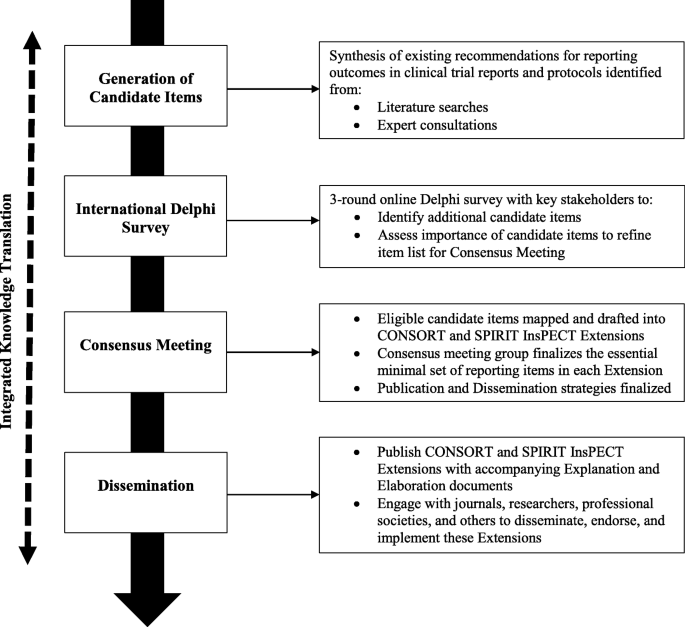
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text

Research2note on Twitter: "The what, when and why of research essential documents @NHSRDForum @dollyblue3 @DigitalCRN @DarkNatter @Research_Innov @WeNurses https://t.co/yPlmOQEHc6" / Twitter

Table 1 from Time-Sinks during the Planning Phase of Investigator Initiated Trials (IITs).Results of an Online Survey | Semantic Scholar