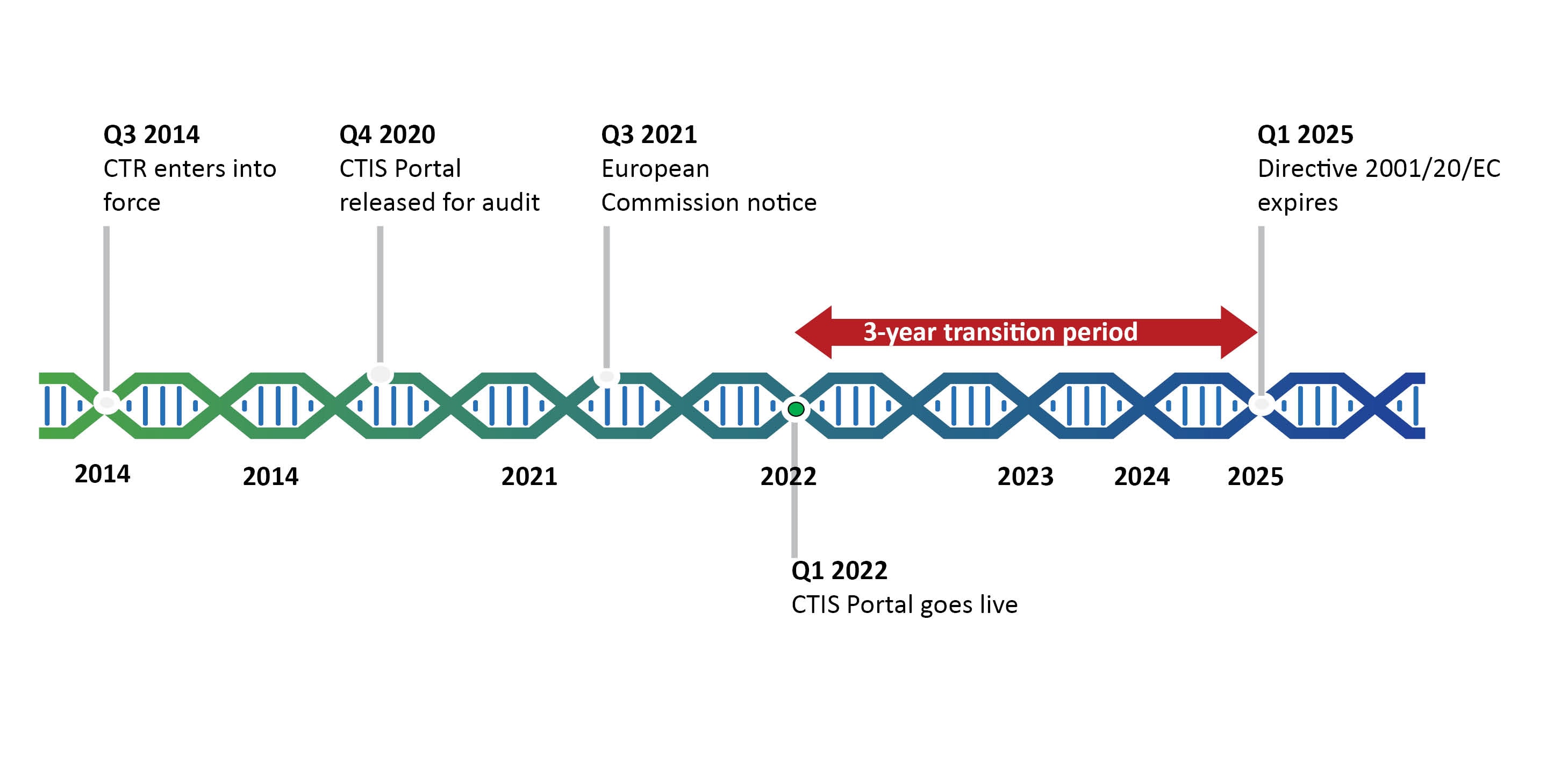
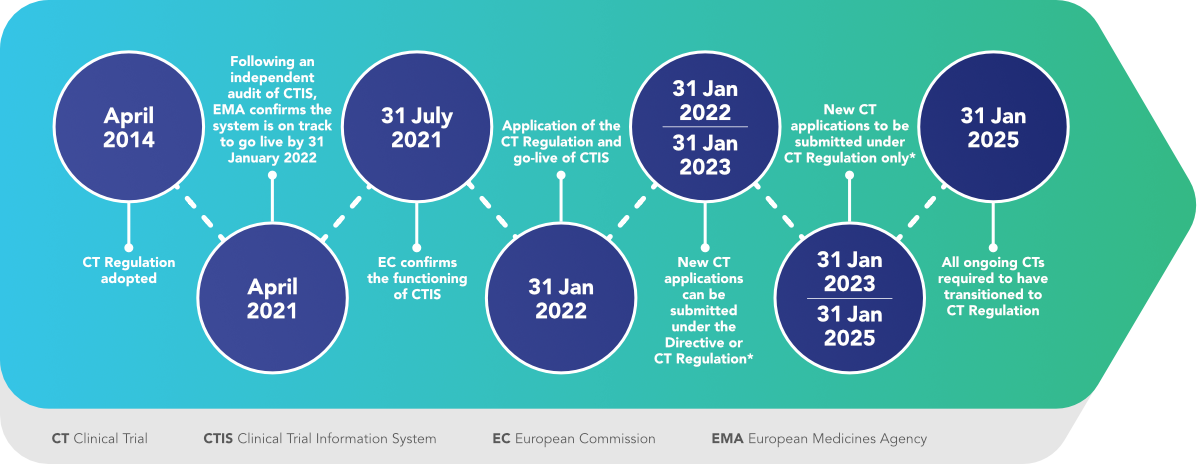
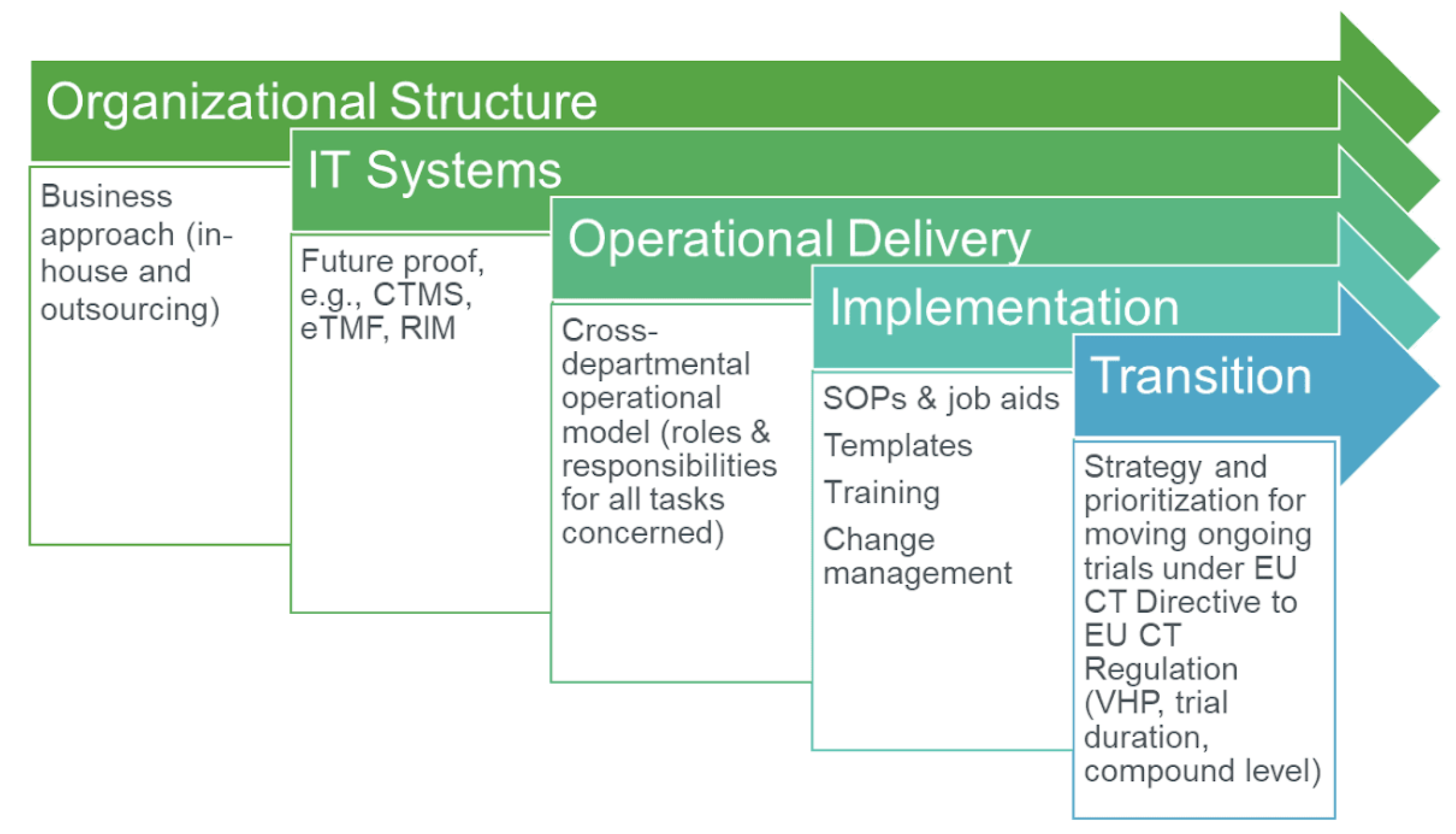
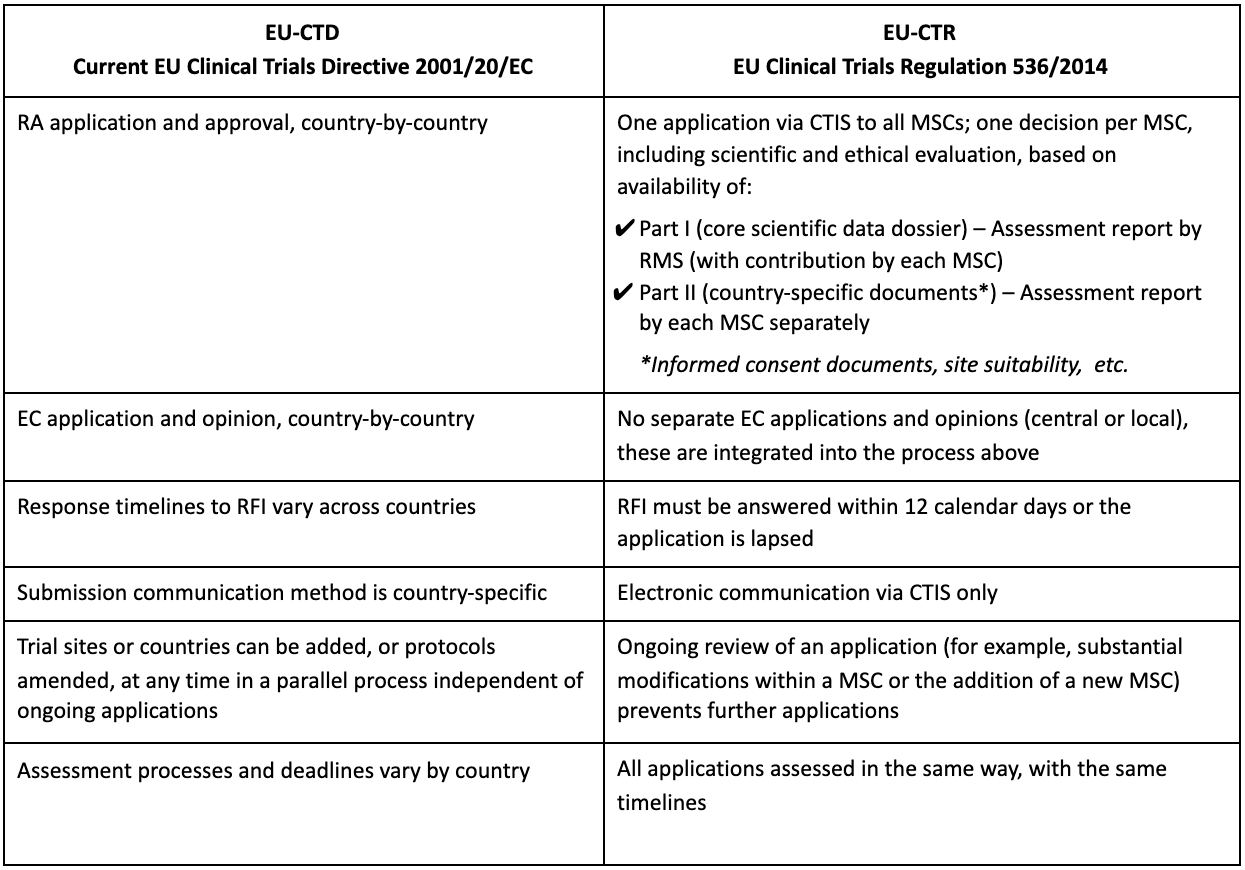
Flow of clinical trials application according to 2001/20/EC Directive.... | Download Scientific Diagram

Book 6: 2023 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library