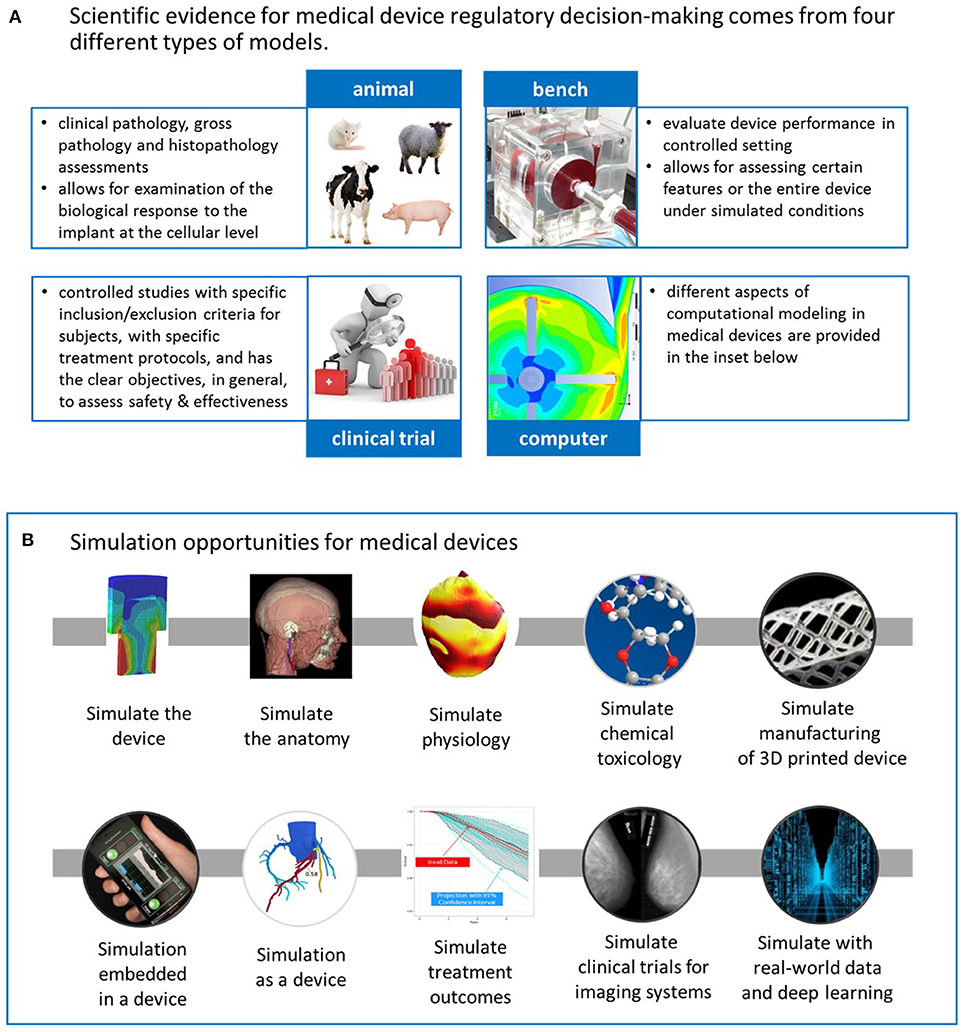
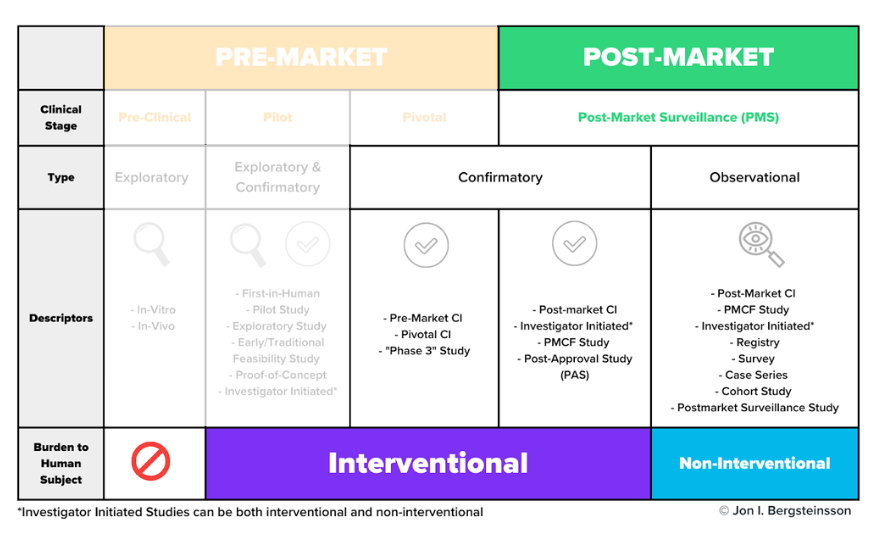
SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch

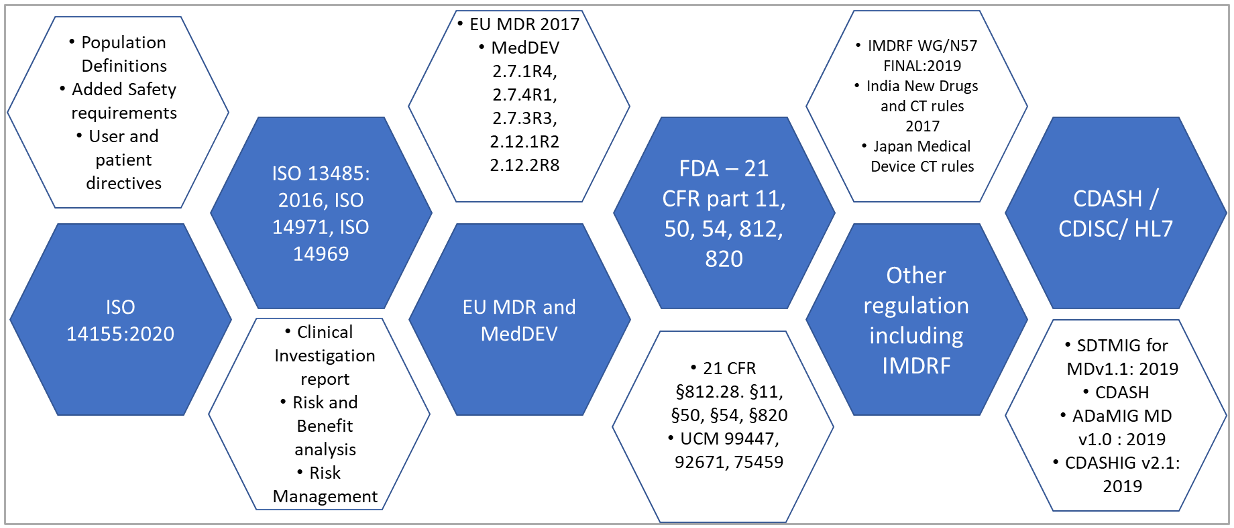
Medical Devices Clinical Evaluation - Summary of Safety and Clinical Performance (SSCP) - Regulation (EU) 2017/745 - GMED Medical Device Certification

Regulation of Medical Devices and their Clinical Trials Studies in the USA: An Update | Bentham Science