After years of lax oversight, the NIH is starting to contact institutions about unreported clinical trial results - STAT

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Months and Severity Score (MOSES) in a Phase III trial (PARCER): A new comprehensive method for reporting adverse events in oncology clinical trials - eClinicalMedicine
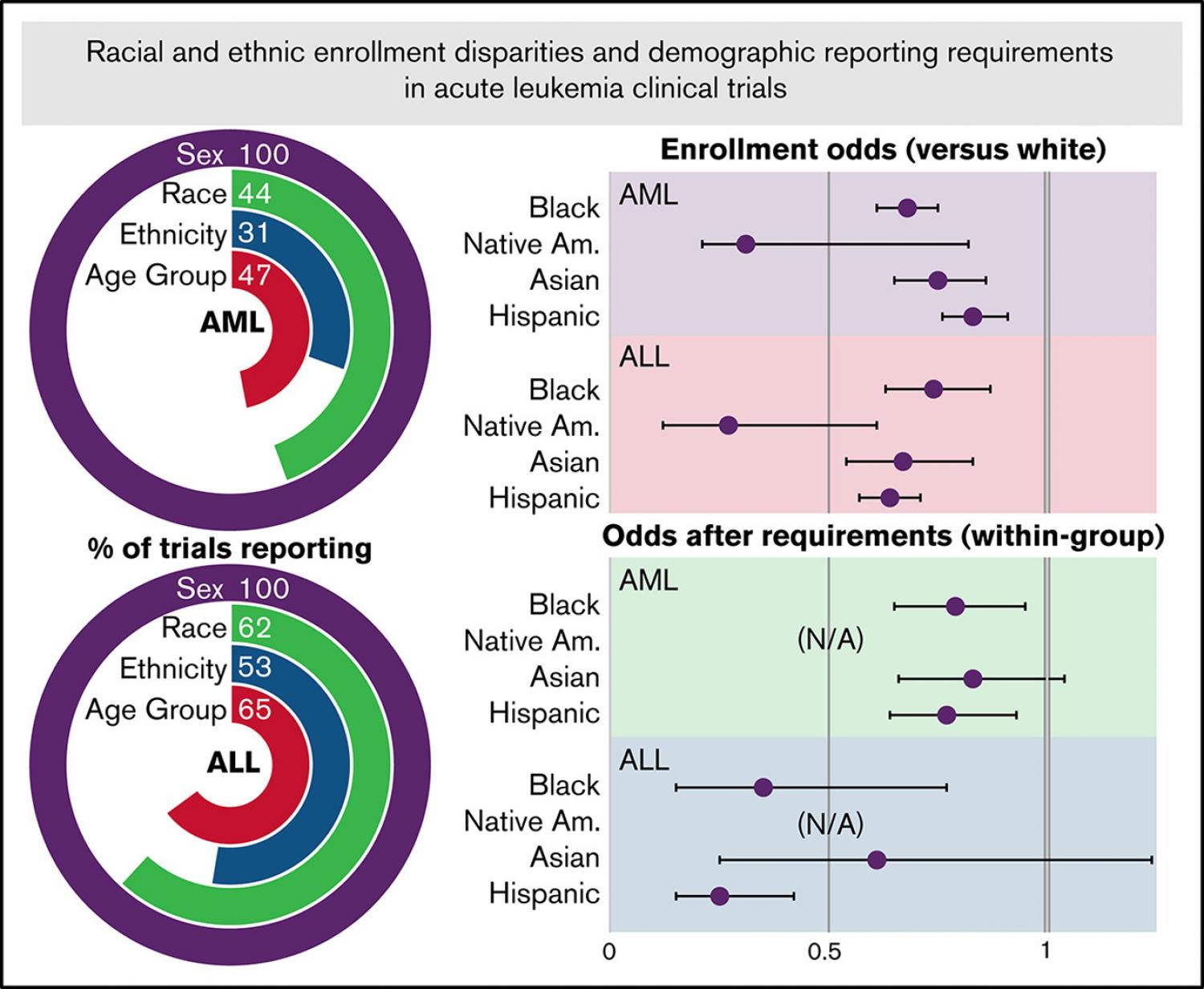
Racial and ethnic enrollment disparities and demographic reporting requirements in acute leukemia clinical trials | Sustainable Development Goals - Resource Centre

Global Healthcare Brand Improves Safety Reporting in Clinical Trials Leveraging Pharmacovigilance Analytics| Quantzig | Business Wire

Clinical Trials: A Practical Guide to Design, Analysis and Reporting: Wang, Duolao: 9781901346725: Amazon.com: Books
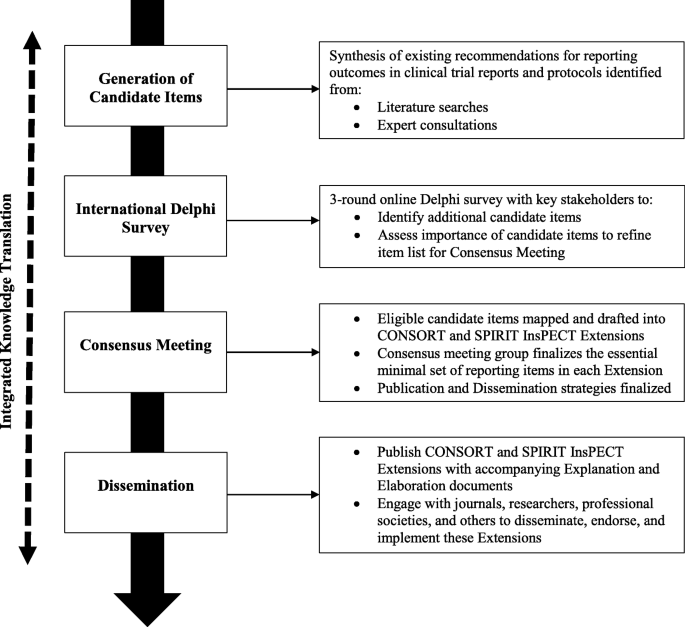
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
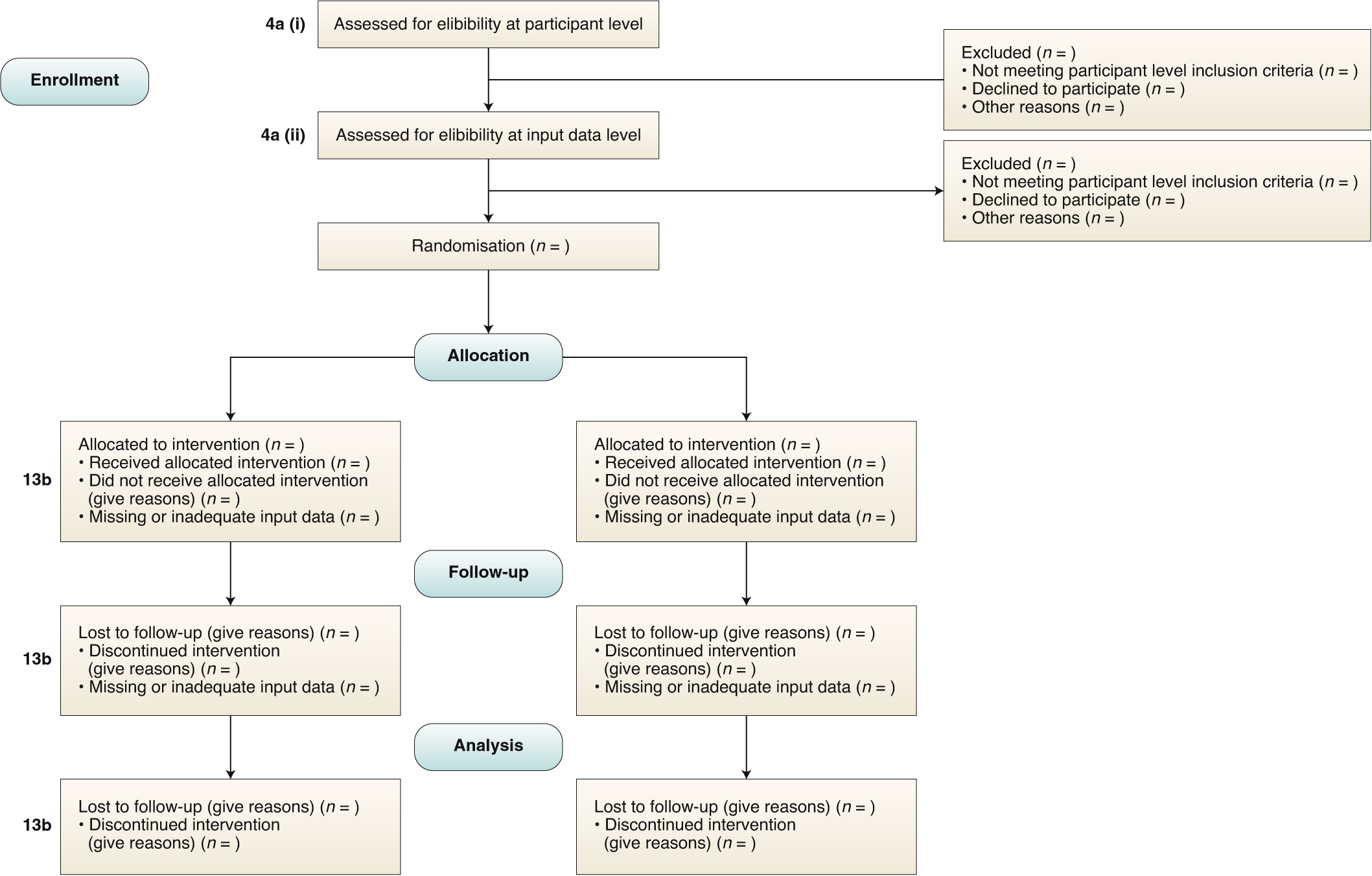
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine

Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning